1. Introduction
Gases, just like liquids, are fluids and can exert forces on objects placed in them. Although air cannot be seen, it has mass, occupies space, and exerts pressure. When an object is placed in air, it experiences an upward force known as upthrust. This force explains why balloons rise, smoke moves upward, and parachutes slow down falling objects.
2. What Is Upthrust?
Upthrust (buoyant force) is the upward force exerted by a fluid (liquid or gas) on an object immersed in it.
In gases, upthrust occurs because air pressure increases with depth. The pressure acting on the bottom of an object is greater than the pressure acting on the top, resulting in a net upward force.
3. Archimedes’ Principle in Gases
Archimedes’ principle also applies to gases and states that:
An object wholly or partially immersed in a gas experiences an upthrust equal to the weight of the gas displaced.
This means the greater the volume of air displaced by an object, the greater the upthrust acting on it.
4. Mathematical Expression for Upthrust in Gases
The upthrust in gases can be calculated using the formula:U=ρgV
Where:
- U = upthrust (N)
- ρ = density of the gas (kg/m³)
- g = acceleration due to gravity (m/s²)
- V = volume of gas displaced (m³)
5. Conditions for Floating, Rising, and Falling in Air

| Comparison of Forces | Result |
|---|---|
| Upthrust > Weight of object | Object rises |
| Upthrust = Weight of object | Object floats |
| Upthrust < Weight of object | Object falls |
6. Illustrations of Upthrust in Gases
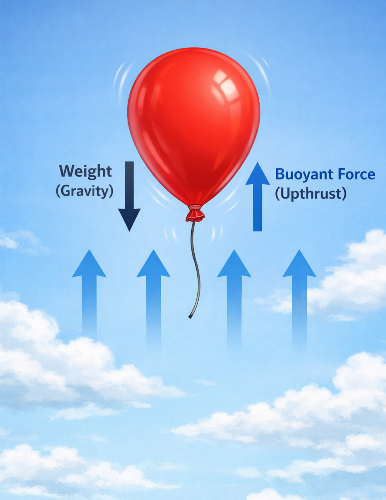
(a) Balloons
A helium or hot-air balloon rises because it displaces air that is denser than itself. The upthrust acting on the balloon is greater than its weight, causing it to rise upward.
(b) Parachutes
When a parachute opens, it increases the surface area and displaces more air. The upthrust (and air resistance) acting upward increases, reducing the downward motion of the parachutist and allowing a safe landing.
(c) Smoke and Hot Air
Hot air and smoke rise because they are less dense than the surrounding cooler air. The upthrust acting on them is greater than their weight.
7. Worked Examples
Example 1:
An object displaces 0.1 m³ of air. Given that its’ density of air = 1.2 kg/m³ and the acceleration due to gravity = 10 m/s². calculate upthrust in air.
Calculate the upthrust acting on the object.
Solution:U=ρgV U=1.2×10×0.1 U=1.2N
Upthrust = 1.2 N
Example 2: Determining Motion of an Object in Air
A balloon has a weight of 0.9 N.
Upthrust acting on it is 1.2 N.
Since:Upthrust>Weight
The balloon will rise.
Example 3: Volume of Air Required for Floating
An object has a weight of 2.4 N.
Density of air = 1.2 kg/m³, g=10m/s2
Calculate the minimum volume of air the object must displace to float.2.4=1.2×10×V V=122.4 V=0.2m3
Required volume = 0.2 m³
8. Problems Involving Upthrust in Gases
- A balloon displaces 0.25 m³ of air.
Density of air = 1.2 kg/m³, g=10m/s2.
Calculate the upthrust acting on the balloon. - An object weighs 3 N and experiences an upthrust of 2 N in air.
State whether the object will rise, float, or fall, giving a reason. - Explain why a parachutist falls slowly after opening a parachute.
- A hot-air balloon displaces 1.5 m³ of air.
Calculate the upthrust acting on the balloon. - State two applications of upthrust in gases in everyday life.
9. Conclusion
Upthrust in gases is an important concept that explains the behavior of objects in air. It depends on the density of the gas, the volume displaced, and gravity. Understanding upthrust helps explain real-life phenomena such as balloons rising, parachutes slowing descent, and hot air moving upward.

Leave a Reply