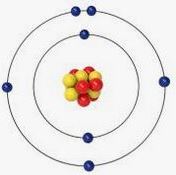
Atomic structure describes how an atom is built from protons, neutrons, and electrons. At the center of the atom is the nucleus, containing positively charged protons and neutral neutrons. Negatively charged electrons orbit the nucleus in shells, with their negative charge attracting the positive protons to hold the atom together. Atoms are electrically neutral because they have an equal number of protons and electrons.
The nucleus of an atom has a specific number of protons and neutrons. The number of protons in the nucleus is called the atomic or proton number. When the number of protons and the number of neutrons in the nucleus are summed up, the resultant number is known as the mass number. Mass number is also known as the nucleon number.

Different atoms has different mass number. For example, hydrogen atom has mass number of 2, meaning it has 1 neutron and 1 proton in it’s nucleus. A neon atom has mass number as 20 having 10 protons, 10 neutrons and 10 electrons. similarly, helium atom has mass number 4 with 2 protons, 2 neutrons and 2 electrons.
describing the mass number in atomic structure
If a certain atom X has atomic number Z with N neutrons and mass number A, then we can express it as:
Thus neon, helium and hydrogen atom will be represents as:
where Ne is neon atom, He is the helium atom and H the hydrogen atom.
There exists atoms that have the same atomic number but with different mass numbers. Such atoms are said to be isotopes. For example carbon-14 and carbon-12 has mass number 14 and 12 respectively but both has atomic mass 6.
The two will be represented as shown:
Stability of the nuclear in atomic structure
A nuclear is said to be stable when a ratio of it’s proton to neutron number is 1 or close to 1. that is
As atoms gets heavier, there is a marked deviation from this ratio, with the neutron number exceeding that of protons. This causes the nucleus to be unstable and hence increases chances of the nuclear disintegrating to gain stability. A graph of number neutrons N against number of protons Z for different nucleus is illustrated below.

From the graph, it is observed that the stable nuclides are outside the stability line.
Nuclides above the stability lines have too many neutrons. Such nuclides decays in such a way that the number protons increases.
Nuclides below the stability line have too many protons . Therefore, they decay to decrease the number of protons.