When radioactive materials undergoes radioactive decay, they produce radiations that exhibits different properties.
There are two broad categories of radiations: Ionizing radiation, which has enough energy to remove electrons from atoms and includes alpha particles, beta particles, neutrons, X-rays, and gamma rays. Non-ionizing radiation, which does not have enough energy to do so and includes radio waves, microwaves, infrared, and visible light. Ionizing radiation is further categorized into particle radiation (alpha, beta, neutrons) and electromagnetic radiation (X-rays, gamma rays).
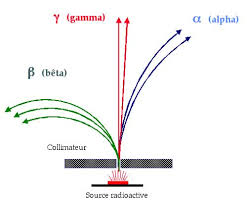
One of the methods we use to distinguish among different radiations is how they behave inside magnetic and electric field. Figure below how a radiations from a radium source are deflected by magnetic field.

The radium source is placed in a thick lead box with a small opening. When a strong magnetic field is introduced perpendicular to the path of radiations, some are deflected . Using Fleming’s left-hand rule, we show that Radiation P is positively charged, R is negatively charged while Q carries no charge.
The positively charged radiation is called the alpha(α) radiations. The negatively charged radiations are referred to as beta (β) radiations.
The uncharged radiations are known as the gamma(γ) radiations.
From the diagram above, alpha particles are deflected the least suggesting that they are the heaviest. Alpha particles are basically helium nucleus

Beta particles are found to be electrons: